- Equipment
- Applications
- Publications
- Contact
- Funding
-
Enzyme activity assays
The facility establishes protocols for quantitative assessment of the enzymatic activity of biologically and/or medically important enzymes, to be used in functional studies and drug screenings.
The scheme below presents a protocol established in BRI for assessing quantitatively the enzymatic activity of PI3Kinase, based on the ELISA principle. The method has been used successfully in drug screenings (see below in section 4, and ref in the end of the page).
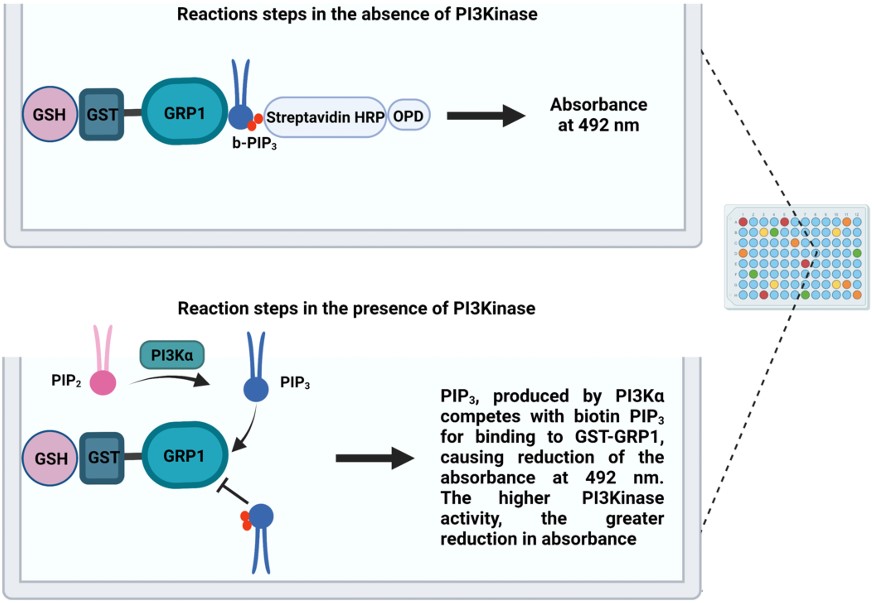
-
In vitro protein-protein interactions
The facility establishes protocols for assessing qualitatively and quantitatively protein-protein interactions, for membrane or soluble proteins.
The scheme below presents a protocol established in BRI for assessing the interaction between MYC and MAX. The method has been used successfully in drug screenings (ongoing study).
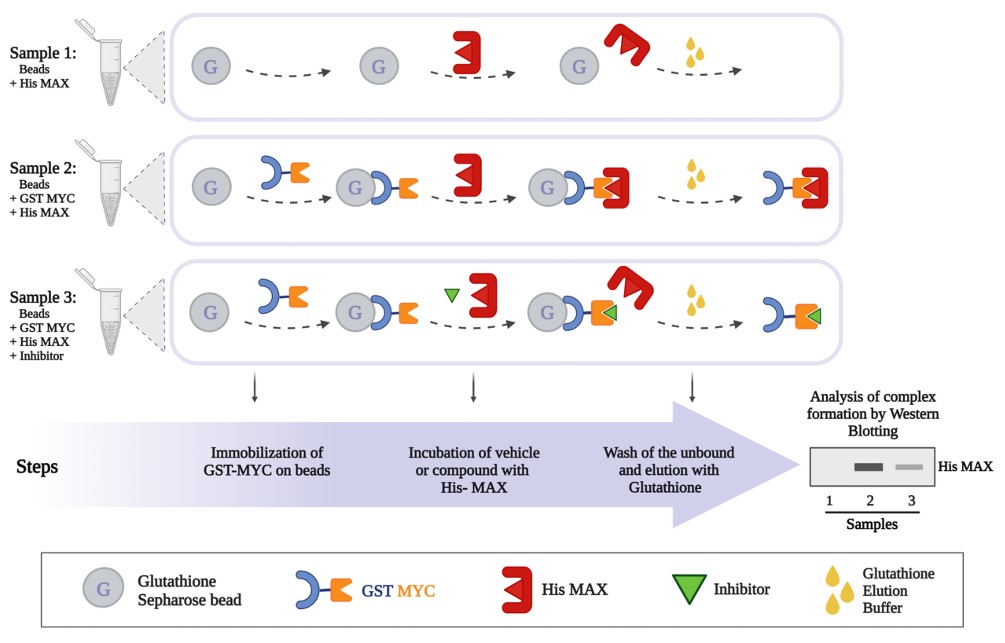
- Savvas Christoforidis, BRI-FORTH & University of Ioannina, Greece
- Anastasia Politou, BRI-FORTH & University of Ioannina, Greece
- Zoe Cournia, Biomedical Research Foundation, Academy of Athens, Greece
- Argiris Efstratiadis, Biomedical Research Foundation, Academy of Athens, Greece
- Apostolos Klinakis, Biomedical Research Foundation, Academy of Athens, Greece
- Konstantinos Tamvakopoulos, Biomedical Research Foundation, Academy of Athens, Greece
- Agianian Bogos, Albert Einstein College of Medicine, New York, USA
- Giorgos Leondaritis, Department of Medicine, University of Ioannina, Greece
- Andreas Tzakos, Department of Chemistry, University of Ioannina, Greece
- Grigoris Zoidis, Department of Pharmacy, National and Kapodistrian University of Athens, Greece.
- Vasiliki Sarli, Department of Chemistry, Aristotle University of Thessaloniki, Greece
The experimental capabilities of our Facility for “Protein Biochemistry and Drug Discovery” span from molecular cloning to the biochemical characterization of target proteins, protein-protein interaction analysis and protein-based drug screenings. Additionally, we have long experience with a wide variety of protein types, including intrinsically disordered proteins, membrane proteins, protein complexes, and soluble proteins.
1. Protein Expression
The PBDD Facility specializes in protein expression and purification by employing three host systems: Escherichia coli bacteria, insect cells (for baculovirus expression) and HEK293 cells (for adenovirus- or transfection- based protein expression), depending on the structural and functional characteristics of the individual protein.

2. Protein Purification
Following harvesting and cell lysis, the expressed proteins are purified using a variety of chromatographic techniques, including affinity, ion exchange, and size exclusion chromatography. We optimize the purification protocol tailored to the specific characteristics of the protein in focus.


All steps of the purification process are analyzed by SDS-PAGE and coomassie blue or silver staining, while the identity of the protein of interest is validated by Western blotting, using specific antibodies.
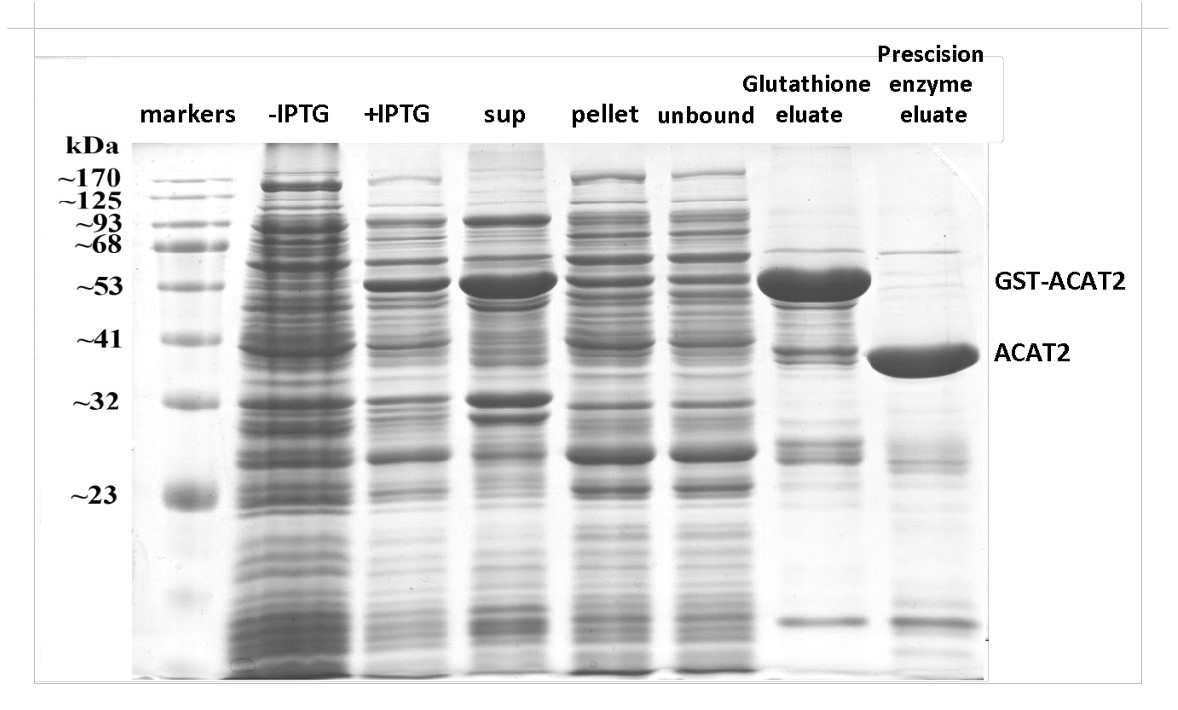
3. Biochemical Support
We provide biochemical support for protein quality control, comprehensive protein characterization, and various interaction studies including:
4. Screening assays, for drug discovery
In the “drug discovery” projects of BRI, we are collaborating with computer scientists, structural biologists, chemists as well as experts in animal models. In this network, we are contributing by setting up in vitro biochemical assays, for screening chemical libraries, to identify novel inhibitors of oncogenic proteins.
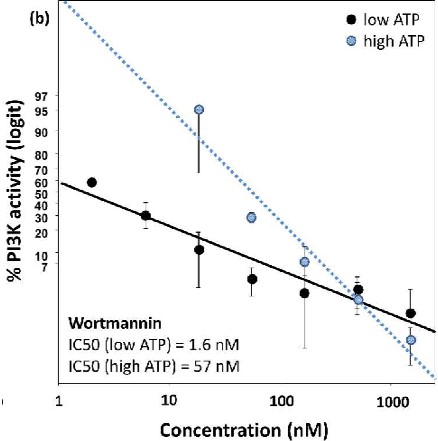
The figure on the right shows a screening experiment of novel chemical compounds, candidate inhibitors against the oncogenic PI3Kinase (PIK3CA) mutated at position H1047R, a known hot spot cancerous mutation.
Upon identification of a novel inhibitor, we proceed in assessing the IC50 value (see example in the figure on the right), in the presence of low or high concentration of the substrate, thus getting insights into the possible allosteric mechanism of inhibition.
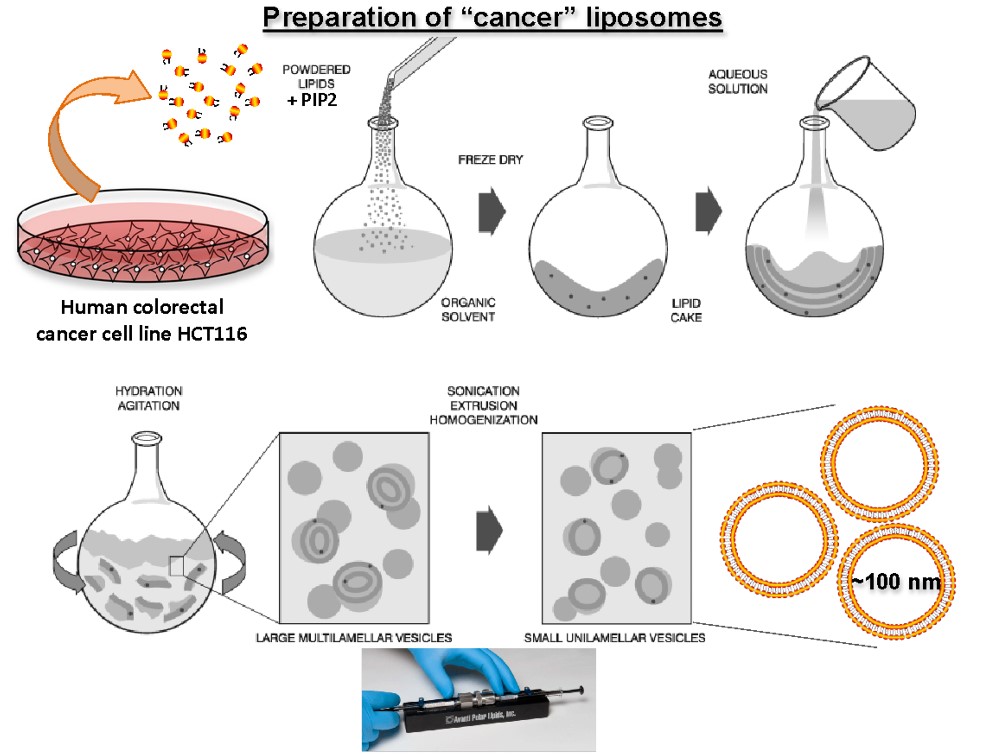
In cases where the screening involves proteins functioning at cellular membranes (eg. RabGTPases, PI3Kinases), we prepare liposomes containing the physiological substrates and the proteins of interest, using in vitro reconstitution approaches (see scheme below), using lipids extracted from cell lines, or completely chemically defined lipids. The Avanti mini-Extruder is used to prepare homogenous (in size) preparation of liposomes.
Finally, besides the above protein-based (cell-free) drug screening, the facility develops in vitro cell-based assays for screening of chemical compounds or natural products for the identification of novel drugs in cancer, cardiovascular and neurodegenerative diseases.
Collaborators in PI3Kinase and MYC-MAX “drug discovery” projects:
Internal Use
Partnership




© Copyright 2025| FOUNDATION FOR RESEARCH & TECHNOLOGY - HELLAS | All rights reserved | Powered by Apogee Information Systems



