- Equipment
- Applications
- Publications
- Contact
- Inverted microscope: DMI6000, Leica
- Five (5) detectors: three PMT’s and two highly sensitive Avalanche Photodiodes (APD) for very low signal imaging.
- Two (2) scanners: a classical line scanner and a resonance scanner for faster imaging.
- Excitation wavelengths:
- ✓ STED laser: 592nm
- ✓ Argon laser: 458, 476, 488, 496, 514 nm
- ✓ DPSS-Laser: 561 nm
- ✓ HeNe laser: 633 nm
- Six (6) objective lenses
- ✓ APO CS 10.0x0.40 DRY UV,
- ✓ APO CS 20.0x0.70 IMM UV,
- ✓ APO CS 40.0x1.25 OIL UV,
- ✓ APO CS 63.0x1.20 WATER UV,
- ✓ APO CS 63.0x1.40 OIL UV and
- ✓ APO CS 100.0x1.40 OIL UV
- Inverted microscope: DMI6000, Leica
- 4 (four) detectors: 2 standard (GaAs) PMTs, 1 high sensitivity (HyD) PMT, 1 PMT for transmitted light
- Two (2) scanners: a classical line scanner and a resonance scanner for faster imaging.
- Excitation wavelengths:
- ✓ Diode laser: 405 nm
- ✓ Argon laser: 458, 476, 488, 496, 514 nm
- ✓ DPSS-Laser: 561 nm
- ✓ HeNe laser: 633 nm
- Six (6) objective lenses
- ✓ APO CS 10.0x0.40 DRY UV,
- ✓ APO CS 20.0x0.70 IMM UV,
- ✓ APO CS 40.0x1.25 OIL UV,
- ✓ APO CS 63.0x1.40 OIL UV and
- ✓ APO CS 100.0x1.40 OIL UV
1. LeicaSP5/STED
This microscope is an inverted fully motorized confocal microscope from Leica (SP5) combined with a super resolution module (Super Resolution Light Microscope), equipped with temperature and CO2-air chamber for live-cell imaging

Figure 1: Leica TCS SP5/STED confocal microscope.
The SP5 is configured with
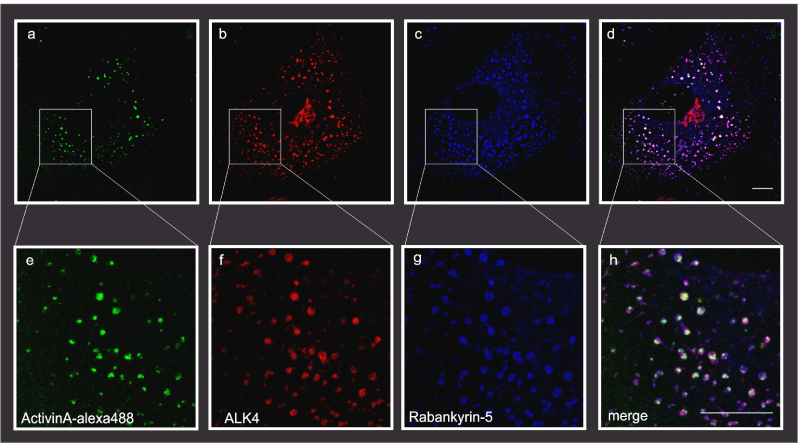
Figure 2: Example of triple immunofluorescence confocal laser scanning microscopy, Scale bar, 10μm (Kostopoulou et al., 2021) (Fotsis and Murphy Group)
Note that the 405 nm laser is not present because its slot is occupied by the CW laser. DAPI should be replaced by DRAQ5 whenever a nuclear staining is needed.
2. Leica TCS SP5 II confocal microscope

Figure 3: Leica TCS SP5 II confocal microscope.
Inverted research microscope Leica DMI6000 CS Life Imaging Services chamber, temperature and CO2-air unit. The SP5 is configured with
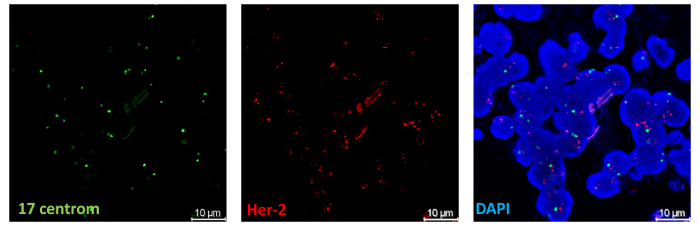
Figure 4: FISH for Her-2 gene amplification in human breast tissues (Lampri Evangeli-Department of Pathology, Medical School University of Ioannina, Greece & Sofia Bellou-Fotsis and Murphy Group)
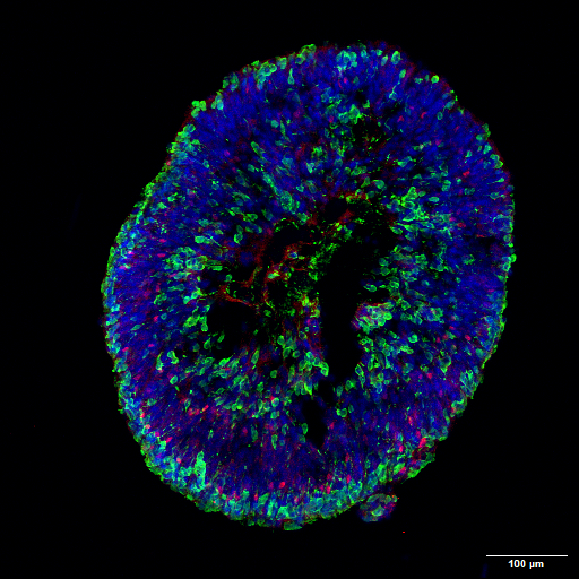
Figure 5: hiPSC- derived Retinal Organoid on Day120 of differentiation expressing photoreceptors (green) and horizontal cells (red). Nuclei stained in blue (Aikaterini Apostolidi & Eleni Bagli- Fotsis and Murphy Lab).
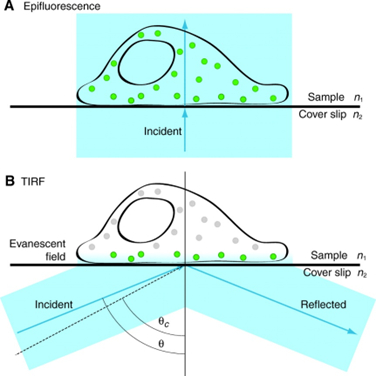
3. TIRF Microscope
Total internal reflection fluorescence microscopy permits high signal-to-noise imaging of fluorescently-labeled molecules at surfaces and interfaces. By adjusting the angle of the illuminating light beyond the critical angle at an interface between media of two different refractive indices (i.e. achieving total internal reflection), a shallow evanescent wave is generated into the medium of lower refractive index that can excite fluorescent molecules that lie within 100 nm of the interface. This results in high signal-to-noise images, due to the lack of signal beyond the 100 nm depth of illumination. This is ideal for studying signal molecule fluorescence, cell-substrate interactions and membrane dynamics (See TIRF video from Basagiannis et al., 2016 - http://www.jbc.org/content/291/32/16892/suppl/DC1).
The facility houses Leica AF 7000 TIRF system equipped with a 100x/1.45 NA Plan Apochromat TIRF objective lens (in addition to 10x, 20x, 40x, and 60x lenses), DIC & phase optics, UV, FITC, TRITC, a low-profile stage incubator (to facilitate live cell imaging) and a CCD camera. The system integrates four wavelengths; 405nm, 488nm, 561nm, and 635nm with fast AOTF control.

Figure 7: Leica AF 7000 TIRF system-Leica DMI 6000B inverted microscope-Pecan Chamber and temperature CO2-air control unit
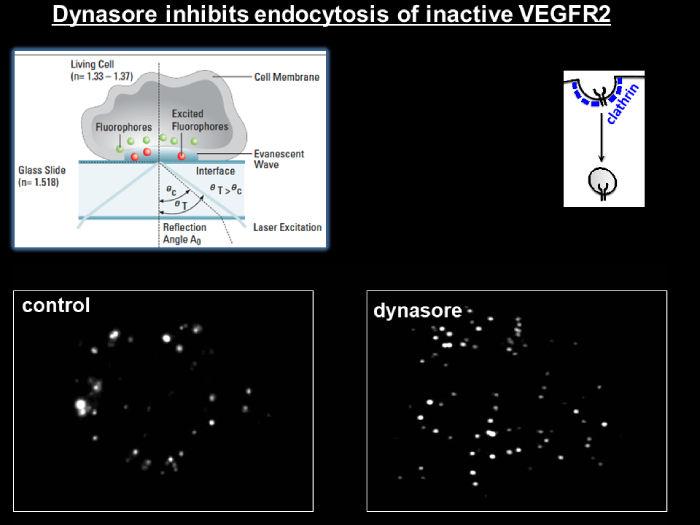
Figure 8: TIRF microscopy for VEGFR2-endocytosis in the absence (left panel) or presence (right panel) of dynasore (Basagiannis et al., 2016) (Christoforidis Lab)
4. IncuCyte ZOOM System
The IncuCyte ZOOM® system is a live-cell imaging and analysis platform that enables automated quantification of cell behavior over time (from hours to weeks) by automatically gathering and analyzing images around the clock. The system provides insight into active biological processes in real-time which is not possible using single-point and end-point measurements. The system resides within the controlled environment of a standard cell incubator. All imaging is completely non-invasive and non-perturbing to cell health. The system can process multiple plates, flasks and dishes in parallel and does not depend on shuttling plates into and out of the incubator.
⬇DOWNLOAD IncuCyte® ZOOM System
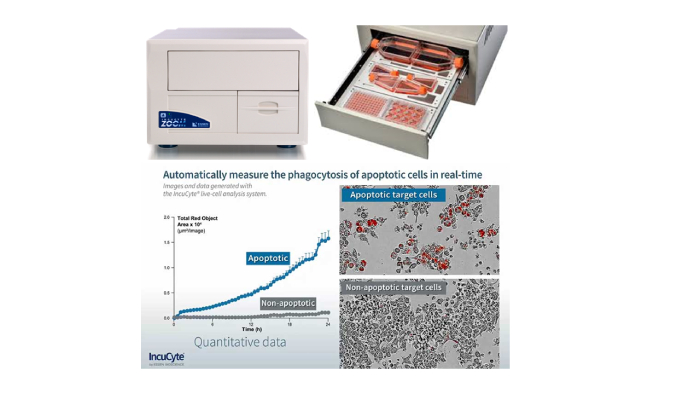
Figure 9: The IncuCyte ZOOM System
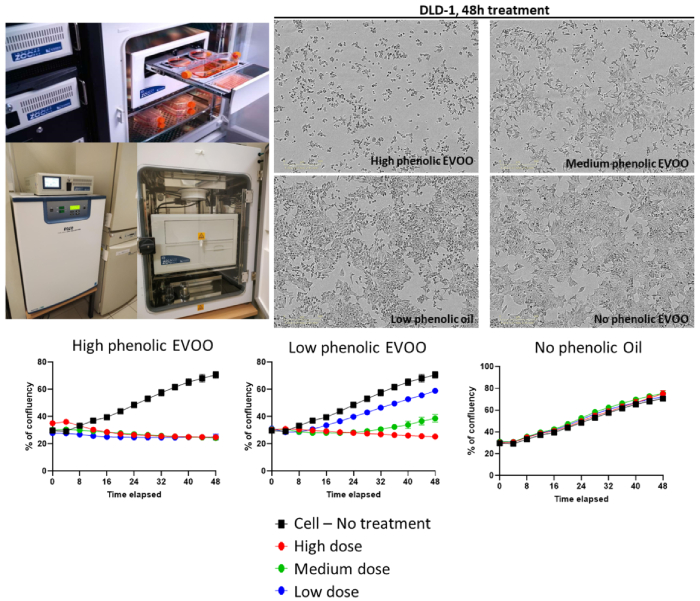
Figure 10:Colorectal adenocarcinoma cell line growth curves in the presence of high phenolic-, low phenolic- or no phenolic-Extra Virgin Olive Oil, followed by IncuCyte (Sofia Bellou, Fotsis and Murphy Lab).
Internal Use
Partnership




© Copyright 2025| FOUNDATION FOR RESEARCH & TECHNOLOGY - HELLAS | All rights reserved | Powered by Apogee Information Systems