- Equipment
- Applications
- Publications
- Contact
In addition to the basic applications, with SP5 we can perform additional microscopy applications. Software wizards for FRAP (Fluorescence Recovery After Photobleaching) and FRET (Fluorescence Resonance Energy Transfer) are available.
1. Super Resolution Light Microscope
The Leica TCS SP5 gated STED CW is a super-resolution confocal microscope. Stimulated Emission Depletion (STED) is a special illumination technique allowing the resolution of sub-cellular details below 80 nm. For STED imaging, a Continuous Wave (CW) laser enables the use of conventional dyes such as Alexa 488, FITC and Oregon Green and established fluorescent proteins such as YFP. Suppression of laser light and improvement of the resolution in the STED mode is achieved by time gating available for the two APDs. STED depletion is done with a 592 nm laser. For excitation in the STED mode the 458 nm, 488 nm, 515 nm can be chosen. This STED setup is ideally suited for one or two color STED with conventional “blue” and “green” dyes including TFP, YFP, and Alexa 488. In addition, our STED is a high-end confocal microscope suitable for most confocal applications.
⬇ DOWNLOAD 2-color STED CW Sample Preparation (Leica)
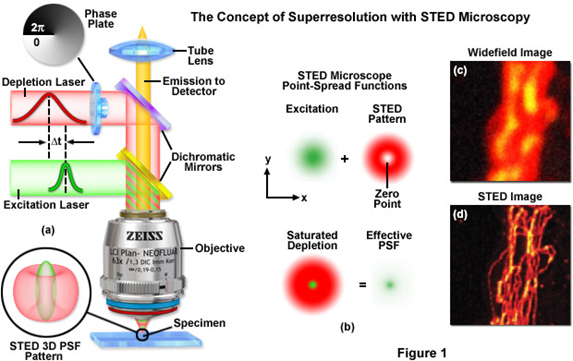
Figure 11: Principle of STED
http://zeiss-campus.magnet.fsu.edu/tutorials/superresolution/stedconcept/indexflash.html
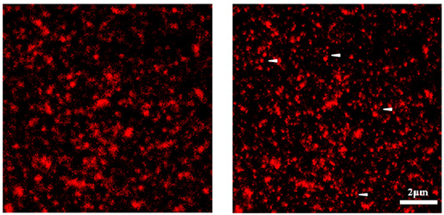
Figure 12: Principle of STED Confocal Laser Scanning Microscopy vs. STED Microscopy (Fotsis and Murphy Group)
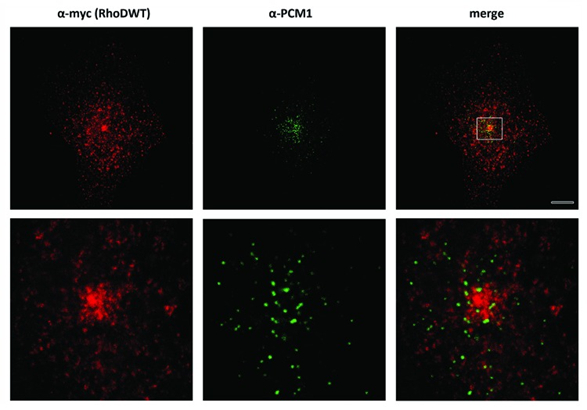
Figure 13: Comparison between Confocal Microscopy (upper panel) and STED Microscopy (lower panel) (Kyrkou et al., 2013) (Fotsis and Murphy Group)
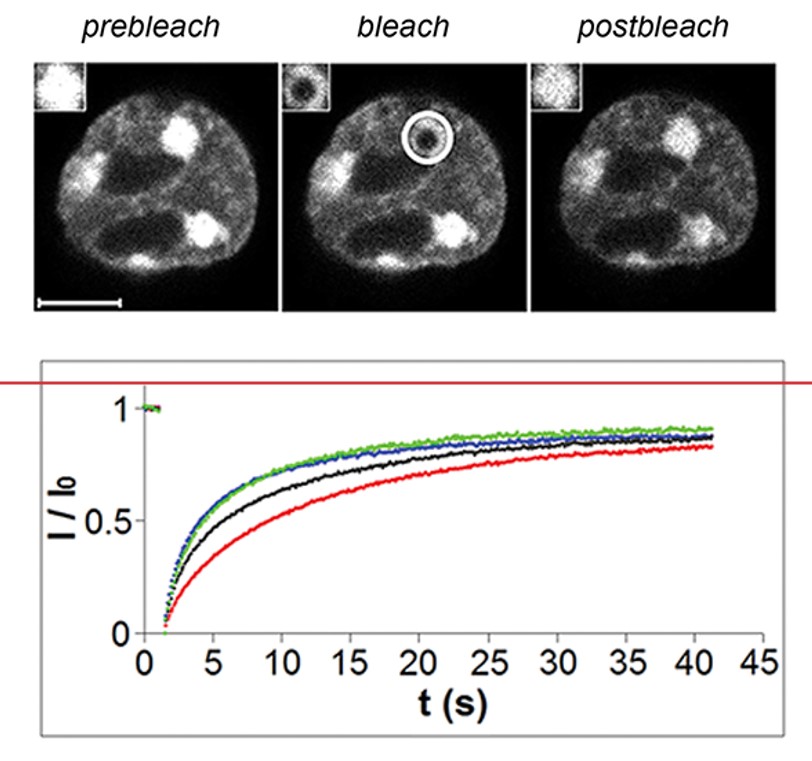
2. FRAP (Fluorescence Recovery After Photobleaching)
Fluorescence recovery after photobleaching (FRAP) has been considered the most widely applied method for observing translational diffusion processes of macromolecules. The resulting information can be used to determine kinetic properties like the diffusion coefficient, mobile fraction and transport rate of the fluorescently labeled molecules.
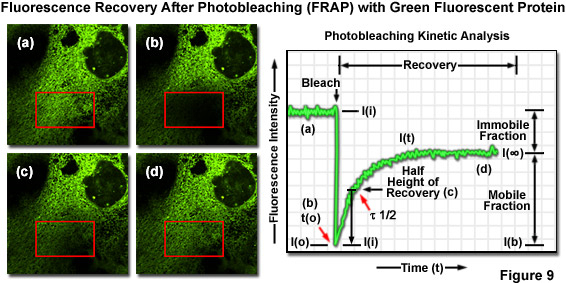
Figure 14: Principle of FRAP
http://zeiss-campus.magnet.fsu.edu/articles/livecellimaging/techniques.html
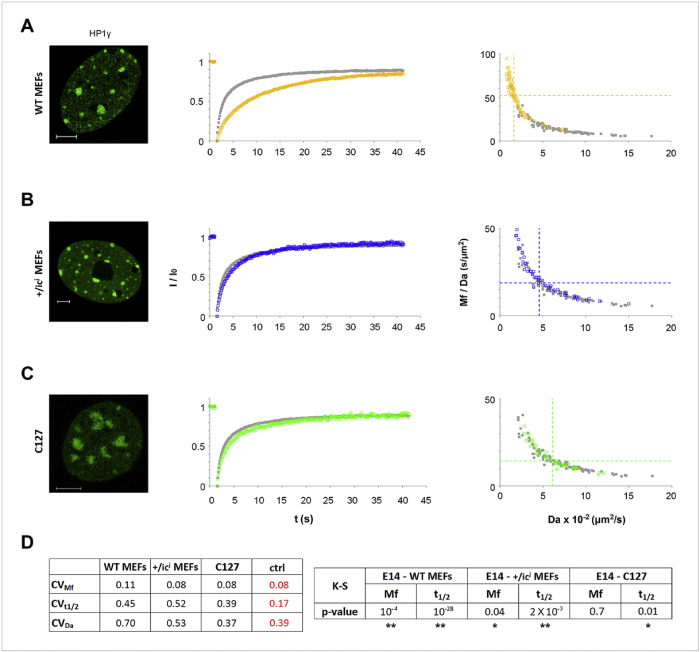
Figure 15: Heterochromatin dynamics in different types of differentiated cells. Scale bar, 5μm (Christogianni et al., 2017) (Georgatos Lab)
3. FRET (Fluorescence Resonance Energy Transfer)
Fluorescence Resonance Energy Transfer (FRET) is a technique, which allows insight into the interactions between proteins or molecules in proximities beyond light microscopic resolution. An excited fluorophore, called the donor, transfers its excited state energy to a light absorbing molecule which is called the acceptor. This transfer of energy is non-radiative. Sensitized Emission is one established method for the evaluation of FRET efficiencies. It can be applied to live cells as well as to fixed samples.
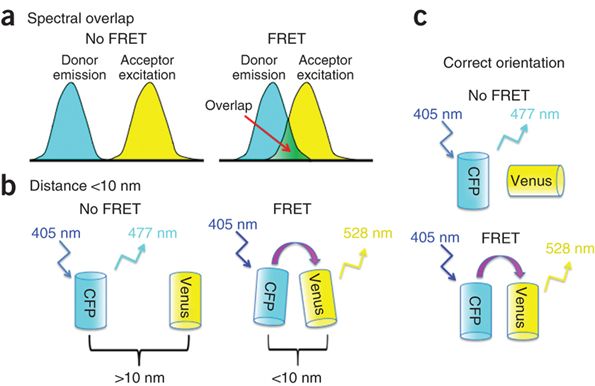
Figure 16: Principle of FRET
https://cam.facilities.northwestern.edu/588-2/fluorescence-resonance-energy-transfer/
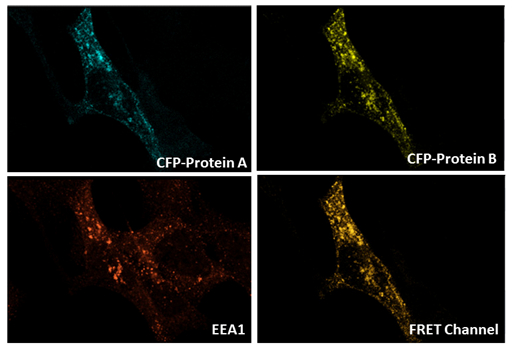
Figure 17: FRET experiment between CFP-Protein A and YFP-Protein B in EEA1-positive vesicle in endothelial cells (Sofia Bellou - Fotsis and Murphy group)
Internal Use
Partnership




© Copyright 2025| FOUNDATION FOR RESEARCH & TECHNOLOGY - HELLAS | All rights reserved | Powered by Apogee Information Systems



